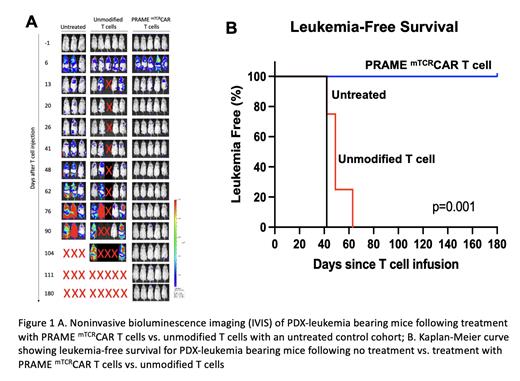
Chimeric antigen receptor (CAR) T cell therapy was a resounding success in CD19+ ALL despite the fact that it is essential for B-cell maturation because it is not expressed during normal myeloid hematopoiesis. Normal and leukemic B-cells are targeted by CD19+ CAR T-cells without causing myeloid hematopoietic toxicity. Advancing such therapy to acute myeloid leukemia (AML) has been challenging in part due to a paucity of “dispensable” targets or targets whose expression is limited to the leukemic cells. Using a large AML and normal hematopoiesis transcriptome database, we pursued discovery of “AML-Restricted Targets”; genes that are silent in normal hematopoiesis but expressed in AML. This broad discovery effort yielded a library of AML-restricted targets that included PRAME (Preferentially Expressed Antigen in Melanoma), an intracellular protein expressed via MHC on the cell surface, to be a highly expressed in AML including the aggressive KMT2A-rearranged (KMT2A-r) AML. We used a TCR mimic (mTCR) antibody, which recognizes the PRAME peptide/HLA-A2 complex on the tumor cell surface, to develop a PRAME directed TCR mimic CAR T (PRAME mTCRCAR T) for pre-clinical studies. Studies in cell lines and CDX models have shown significant efficacy of this CAR T (Kirkey, Bld Adv. 2022). To conduct final IND-enabling studies, we generated a KMT2A-r HLA-A2+/PRAME+ patient-derived xenograft (PDX) model to treat with PRAME mTCRCAR T-cells. Here we demonstrate the in vivo activity of PRAME mTCRCAR T cells against this unique PDX AML model. VL and VH sequences from the PRAME specific TCR mimic antibody (Pr20) were used to construct the single-chain fragment variable domain into the 41-BB/CD3ζ CAR vector. The PDX model was derived from a PRAME +/HLA-A2 + pediatric patient with KMTA2-r AML. PDX cells were transduced with lentiviral ffluciferase for noninvasive bioluminescent IVIS imaging to monitor leukemic progression. Mice were transplanted with 1x10 6 PDX leukemia cells then 1 week later, PDX leukemia-bearing mice were treated with unmodified T cells or PRAME mTCRCAR T cells at 5x10 6 cells (1:1 CD4:CD8) per mouse. Leukemia burden was measured by IVIS imaging and regular peripheral blood analysis.
Mice who received unmodified T cells had rapid disease progression by day 50 and all died by day 105. In contrast, PRAME mTCRCAR T-treated mice rapidly cleared disease and all remained alive and leukemia-free >180 days post-treatment (p=0.001). Average leukemia burden in the control cohort was 3.5% at week 6 and 41.75% at week 11, while no leukemia was detected following CAR T-cell treatment. In addition, the control arm had a marked expansion of leukemia with a 7.75- and 429.6-fold increase in radiance by IVIS at 6 and 11 weeks post-treatment, respectively. No significant increase in radiance was seen in the PRAME mTCRCAR T cell treated group.
We further evaluated hematopoietic toxicity of PRAME mTCRCAR T cells in humanized mice. Sub-lethally irradiated NSG-SGM3 mice were reconstituted with HLA-A2+ CD34 selected human cord blood stem cells with simultaneous and allowed to engraft. Human hematopoietic cell engraftment was evaluated following treatment with unmodified T cells vs. PRAME mTCRCAR T cells. Evaluation of human CD45+ cells in the peripheral blood via flow cytometry showed no difference in the levels of hematopoietic engraftment in mice treated with PRAME mTCRCAR T cell (8%) vs. unmodified T cells (6%) vs. an untreated control cohort (5%), demonstrating lack of toxicity of PRAME mTCRCAR T cells against HLA-A2 hematopoiesis.
We demonstrate that PRAME mTCRCAR T cells can irradicate AML in a target specific manner in aggressive KMT2A-r AML without toxicity. We show potent efficacy with eradication of leukemia in PDX-bearing mice following treatment with PRAME mTCRCAR T cells resulting in prolonged survival, without hematopoietic toxicity. These results provide strong rationale for advancing this therapeutic approach to clinical development.
Disclosures
Pardo:Hematologics Inc: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal